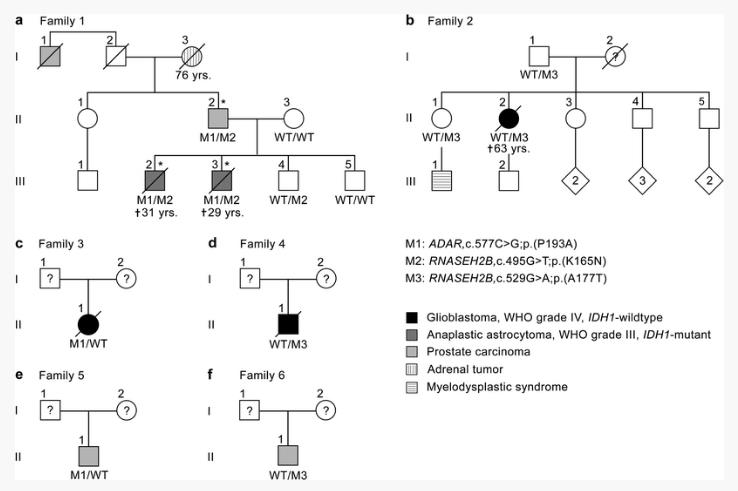
Aicardi-Goutières syndrome (AGS) is a genetically induced inflammatory disease particularly of the brain, which has not been associated with a consistently increased cancer risk to date. In Oct 13, 2017, Ulrike Beyer and others published an article in << Acta Neuropathologica >> which title is“Rare ADAR and RNASEH2B variants and a type I interferon signature in glioma and prostate carcinoma risk and tumorigenesis”. They studied a family with two brothers affected by anaplastic gliomas, and their father and paternal great-uncle diagnosed with prostate carcinoma. In this family, whole-exome sequencing yielded rare, simultaneously heterozygous variants in the Aicardi-Goutières syndrome (AGS) genes ADAR and RNASEH2B co-segregating with the tumor phenotype. By targeted sequencing, they identified novel ADAR and RNASEH2B variants, and a 3- to 17-fold frequency increase of the AGS mutations ADAR,c.577C>G;p.(P193A) and RNASEH2B,c.529G>A;p.(A177T) in the germline of familial glioma patients as well as in test and validation cohorts of glioblastomas and prostate carcinomas versus ethnicity-matched controls, whereby rare RNASEH2B variants were significantly more frequent in familial glioma patients. Their data implicate rare variants in the AGS genes ADAR and RNASEH2B and a type I interferon signature in glioma and prostate carcinoma risk and tumorigenesis, consistent with a genetic basis underlying inflammation-driven malignant transformation in glioma and prostate carcinoma development.
Read More